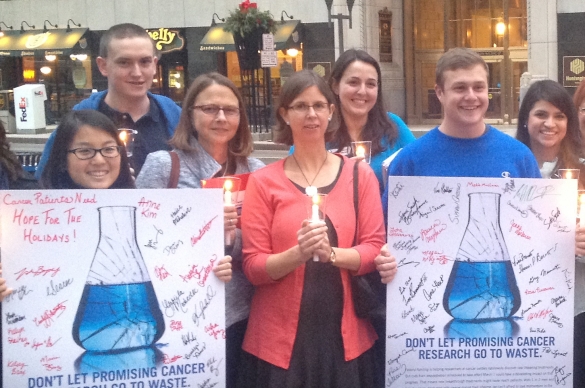Improvements in outcomes for cancer patients require continued research and innovation. ACS CAN advocates for robust federal funding for cancer research, as well as research and drug approval policies that accelerate the development of new treatments while still ensuring patient safety.